The UK evaluates expensive drugs for cost-effectiveness primarily through the National Institute for Health and Care Excellence (NICE) and other bodies like the Scottish Medicines Consortium (SMC). The evaluation is based on clinical effectiveness, cost-effectiveness, and impact on NHS resources.
Key Aspects of Evaluation
1. Cost-Effectiveness Analysis (CEA) using QALYs
- NICE uses the Quality-Adjusted Life Year (QALY) as a measure to assess whether a drug provides sufficient health benefits relative to its cost.

- If the ICER is below £20,000–£30,000 per QALY, the drug is typically considered cost-effective.
- For certain severe or rare diseases, NICE may allow a higher cost per QALY threshold (e.g., up to £100,000 for very rare conditions under the Highly Specialised Technologies (HST) program).
2. Budget Impact Test
- If a drug is expected to cost the NHS more than £20 million per year, NICE may negotiate with the manufacturer for a Managed Access Agreement (MAA) or phased introduction to spread costs.
3. Clinical Evidence & Real-World Data
- NICE considers clinical trial data, real-world effectiveness, and patient-reported outcomes.
- The NHS Commercial Medicines Directorate may negotiate confidential pricing agreements (e.g., rebates or discounts).
4. NHS England & Special Cases
- For cancer drugs, the Cancer Drugs Fund (CDF) allows faster access while gathering more real-world data.
- The Innovative Medicines Fund (IMF) supports non-cancer drugs with promising early data but uncertain long-term benefits.
5. Scotland & Wales
- The Scottish Medicines Consortium (SMC) and the All Wales Medicines Strategy Group (AWMSG) perform similar cost-effectiveness evaluations for their health systems.
Example: Cost-Effectiveness Evaluation of Omalizumab in the UK
Omalizumab (Xolair) is a monoclonal antibody used for severe allergic asthma and chronic spontaneous urticaria (CSU). Its cost-effectiveness for NHS use was evaluated by NICE based on clinical benefit, quality of life improvement, and economic impact.
1. How is the benefit of Omalizumab calculated?
A. Clinical Benefits (Health Gains)
- Clinical trials show reduced asthma exacerbations, hospitalizations, and improved symptom control.
- Fewer oral corticosteroid (OCS) bursts, reducing side effects (osteoporosis, diabetes risk).
- Improved quality of life due to fewer symptoms and better lung function.
B. Quality-Adjusted Life Years (QALY) Calculation
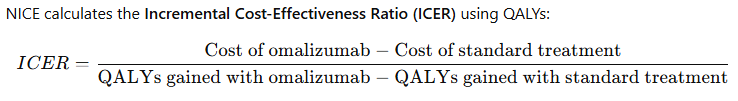
- Without treatment: Patients may experience frequent asthma attacks, reliance on oral corticosteroids, and reduced quality of life (e.g., QALY score = 0.50).
- With Omalizumab: Patients have fewer exacerbations, reduced hospital stays, and improved daily function (e.g., QALY score = 0.72).
- QALYs gained = 0.72 – 0.50 = 0.22**
**NOTE that for Omalizumab initial ICER calculation would have been £8000/26000 (ie. cost of drug) divided by 0.22 (QALY gained) which equals £40-100 000 ie. well above the usual approval threshold of £20-30 000. NHS presumably negotiated a way around that problem to allow approval of Omalizumab.
2. Cost-Effectiveness of Omalizumab
A. NHS Cost Evaluation
- Omalizumab cost: ~£8,000–£26,000 per patient per year (depending on dosage).
- Cost savings: Fewer hospitalizations, ICU admissions, and OCS-related complications.
- NICE’s ICER threshold is £20,000–£30,000 per QALY.
B. NICE Decision on Cost-Effectiveness
- For severe allergic asthma: Approved, as the ICER was ~£28,000 per QALY, within the acceptable NHS threshold.
- For chronic urticaria: Initially not approved due to an ICER > £50,000 per QALY, but later funded under special circumstances.
3. Special NHS Funding Mechanisms
- Managed Access Agreements (MAA): Discounted pricing for eligible patients.
- Real-World Data Collection: Continued monitoring of benefits via the Severe Asthma Registry.
Share this post
Latest News posts
Do you need a Patient Information Leaflet for your medication?
September 26, 2023
Understanding Sepsis: A Patient’s Guide
September 26, 2023
Martha’s Rule: A Lifeline for Patients and Families in the NHS
September 6, 2023
NHS Complaint Procedures
September 4, 2023
Accessing GP Services: A Detailed Overview
August 31, 2023
Unvalidated Laboratory Testing
August 2, 2023
Osteoporosis (Thinning bones)
August 1, 2023
Understanding a Fever
July 31, 2023
Living with & Managing Chronic Illness
July 27, 2023
News archive
- Antifungals in development
- COVID-19
- Events
- Fundraising
- General interest
- How do I...?
- Information and Learning
- Latest research news
- Lifestyle and Coping Skills
- Living with Aspergillosis
- NAC announcements
- News archive
- Patient and Carer Blog
- Patient stories
- Recordings
- Supplements and complementary therapies
- Types of aspergillosis
- Video
